Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Pd in Pd purified by selective precipitation from spent nuclear fuel by laser ablation ICP-MS
Pd in Pd purified by selective precipitation from spent nuclear fuel by laser ablation ICP-MSAsai, Shiho; Ohata, Masaki*; Yomogida, Takumi; Saeki, Morihisa*; Oba, Hironori*; Hanzawa, Yukiko; Horita, Takuma; Kitatsuji, Yoshihiro
Analytical and Bioanalytical Chemistry, 411(5), p.973 - 983, 2019/02
Times Cited Count:11 Percentile:60.2(Biochemical Research Methods)Determination of radiopalladium  Pd is required for ensuring the radiation safety of Pd extracted from spent nuclear fuel for recycling or disposal. We employed laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to simplify an analytical procedure of
Pd is required for ensuring the radiation safety of Pd extracted from spent nuclear fuel for recycling or disposal. We employed laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to simplify an analytical procedure of  Pd. Pd was separated through selective Pd precipitation reaction from spent nuclear fuel. Laser ablation allows direct measurement of the Pd precipitates, skipping the dissolution and dilution procedure. In this study,
Pd. Pd was separated through selective Pd precipitation reaction from spent nuclear fuel. Laser ablation allows direct measurement of the Pd precipitates, skipping the dissolution and dilution procedure. In this study, 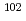 Pd in natural Pd standard solution was used as an internal standard, taking advantage of its absence in spent nuclear fuel. The Pd precipitate was uniformly embedded on the surface of the centrifugal filter, forming a microscopically thin flat surface of Pd. The resulting homogeneous Pd layer is suitable for obtaining a stable signal ratio of
Pd in natural Pd standard solution was used as an internal standard, taking advantage of its absence in spent nuclear fuel. The Pd precipitate was uniformly embedded on the surface of the centrifugal filter, forming a microscopically thin flat surface of Pd. The resulting homogeneous Pd layer is suitable for obtaining a stable signal ratio of  Pd/
Pd/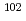 Pd. The amount of
Pd. The amount of  Pd obtained by LA-ICP-MS corresponds to the values obtained by conventional solution nebulization measurement.
Pd obtained by LA-ICP-MS corresponds to the values obtained by conventional solution nebulization measurement.
Miyamoto, Yutaka; Suzuki, Daisuke; Esaka, Fumitaka; Magara, Masaaki
Analytical and Bioanalytical Chemistry, 407(23), p.7165 - 7173, 2015/09
Times Cited Count:8 Percentile:31.37(Biochemical Research Methods)Age of individual uranium-plutonium mixed particles with various U/Pu atomic ratios were determined by inductively-coupled plasma mass spectrometry. Micron-sized particles were prepared from U and Pu certified reference materials. The Pu reference was stored for 4-6 years since the last purification. The Pu purification age was obtained from the  Am/
Am/ Pu ratio which was calculated from the product of three measured ratios of Pu and Am isotopes in the eluted fractions. Am, U and Pu in a sample solution were sequentially separated a small anion-exchange column. The
Pu ratio which was calculated from the product of three measured ratios of Pu and Am isotopes in the eluted fractions. Am, U and Pu in a sample solution were sequentially separated a small anion-exchange column. The  Am/
Am/ Pu ratio was accurately determined by spiking pure
Pu ratio was accurately determined by spiking pure  Am to the sample solution. The determined age of particles with various U/Pu ratios was in good agreement with the expected age with high accuracy and high precision.
Am to the sample solution. The determined age of particles with various U/Pu ratios was in good agreement with the expected age with high accuracy and high precision.
Watanabe, Yoko; Kuwabara, Jun
Analytical and Bioanalytical Chemistry, 384(2), p.547 - 550, 2006/01
Times Cited Count:3 Percentile:11.31(Biochemical Research Methods)In order to reduce the color quenching in the measurement of tritium in urine by liquid scintillation counter (LSC), ultraviolet (UV) irradiation was applied to decompose the organic substances in the sample. Urine was decolorized under UV irradiation in the presence of hydrogen peroxide. As a result, color quenching was considerably suppressed and higher counting efficiency of tritium was obtained. This UV treatment made it possible to increase the urine content in the sample from 2% to 40%(v/v) without significant decrease of counting efficiency. Either higher sensitivity or shorter analysis time was achieved in the tritium measurement by the augumentation of urine content. When the measurement time was 30 min, the detection limit of tritium defined as 3s was 0.03 Bq/ml. At the expense of some sensitivity (set at detection limit of 0.3 Bq/ml), the measurement time was shortened to 0.5 min. These results will make a great improvement to routine tritium monitoring as well as to emergency monitoring in mass tritium exposure.
Ozaki, Takuo; Ambe, Shizuko*; Abe, Tomoko*; Francis, A. J.
Analytical and Bioanalytical Chemistry, 375(4), p.505 - 510, 2003/02
Times Cited Count:7 Percentile:23.17(Biochemical Research Methods)no abstracts in English
Ozaki, Takuo; Arisaka, Makoto; Kimura, Takaumi; Francis, J. A.*; Yoshida, Zenko
Analytical and Bioanalytical Chemistry, 374(6), p.1101 - 1104, 2002/11
Times Cited Count:24 Percentile:57.86(Biochemical Research Methods)no abstracts in English
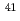 Ca in biological-shield concrete by low-energy X-ray spectrometry
Ca in biological-shield concrete by low-energy X-ray spectrometryIto, Mitsuo; Watanabe, Kazuo; Hatakeyama, Mutsuo; Tachibana, Mitsuo
Analytical and Bioanalytical Chemistry, 372(5-6), p.532 - 536, 2002/03
Times Cited Count:16 Percentile:44.74(Biochemical Research Methods)An X-ray spectrometry method has been developed for the determination of Ca-41 in the biological shield concrete of nuclear reactors. To isolate Ca from other elements, the concrete sample was first decomposed with nitric, hydrofluoric and perchloric acids. Calcium was then separated from other coexisting radionuclides by ion-exchange chromatography and recovered as an oxalate precipitate. X rays at 3.3 keV from Ca-41 in the calcium oxalate pellet were measured. Detection efficiency of the X-ray measurement at 3.3 keV was calculated from those obtained by measuring Fe-55 standard pellets at 5.9 keV using mass absorption coefficients of the calcium oxalate pellet at each X-ray energy value. A lower limit of determination of 8 Bq g-1 was obtained for a sample weight of 1 g.